We at Applied BioAnalytics know that each new generation therapeutics is unique, as are the teams developing them. Our team serve our clients with expert support that fit their bioanalytical needs with cutting edge technology. We provide our clients with comprehensive solutions together with reliable data. We offer personalized and comprehensive services based on, industrial standard procedures and detailed knowledge of regulatory requirements. We at the same time, are ready to address each project’s unique needs by being ready to develop new methodologies and finding novel solutions.
Method Development & Qualification for Pharma and Biopharma Industries
With close collaboration of our clients, Applied BioAnalytics offers custom development of new methods for CZE, CGE/CE-SDS as well as the optimization of existing protocols based on specific requirements. As the analytical needs of any new product’s development phase is hardly predictable, contracting for such projects requires thorough discussion. Our team is dedicated to working closely with our clients throughout the process to ensure each unique project’s successful outcome. In the fields of glycan and protein characterization, our staff have recognizable track records:
-
N-Linked Oligosaccharide Profiling by CE-LIF
-
Quantitative Monosaccharide Analysis via CE-LIF
-
Quantitative Sialic Acid Speciation via CE-LIF
-
Exoglycosidase mediated glycan sequencing / Mapping via CE-LIF
-
Size heterogeneity and purity assessment by CE-SDS
-
Charge variant analysis via cIEF
-
Charge heterogeneity analysis by CZE
Example 1 – High resolution glycan profiling
N-glycan composition profiling is essential for quality testing of biotherapeutics because the glycan structure is designated as a critical quality attribute (CQA) that must be monitored during manufacturing. The glycan profile of a finished product is used to assess the consistency of a manufacturing process from batch to batch. Our capillary electrophoresis separation-based glycan profiling technology enables extraordinary resolution, which cannot be addressed by LC methods alone.
Mol Cell Proteomics. 2019 Dec;18(12):2524-2531
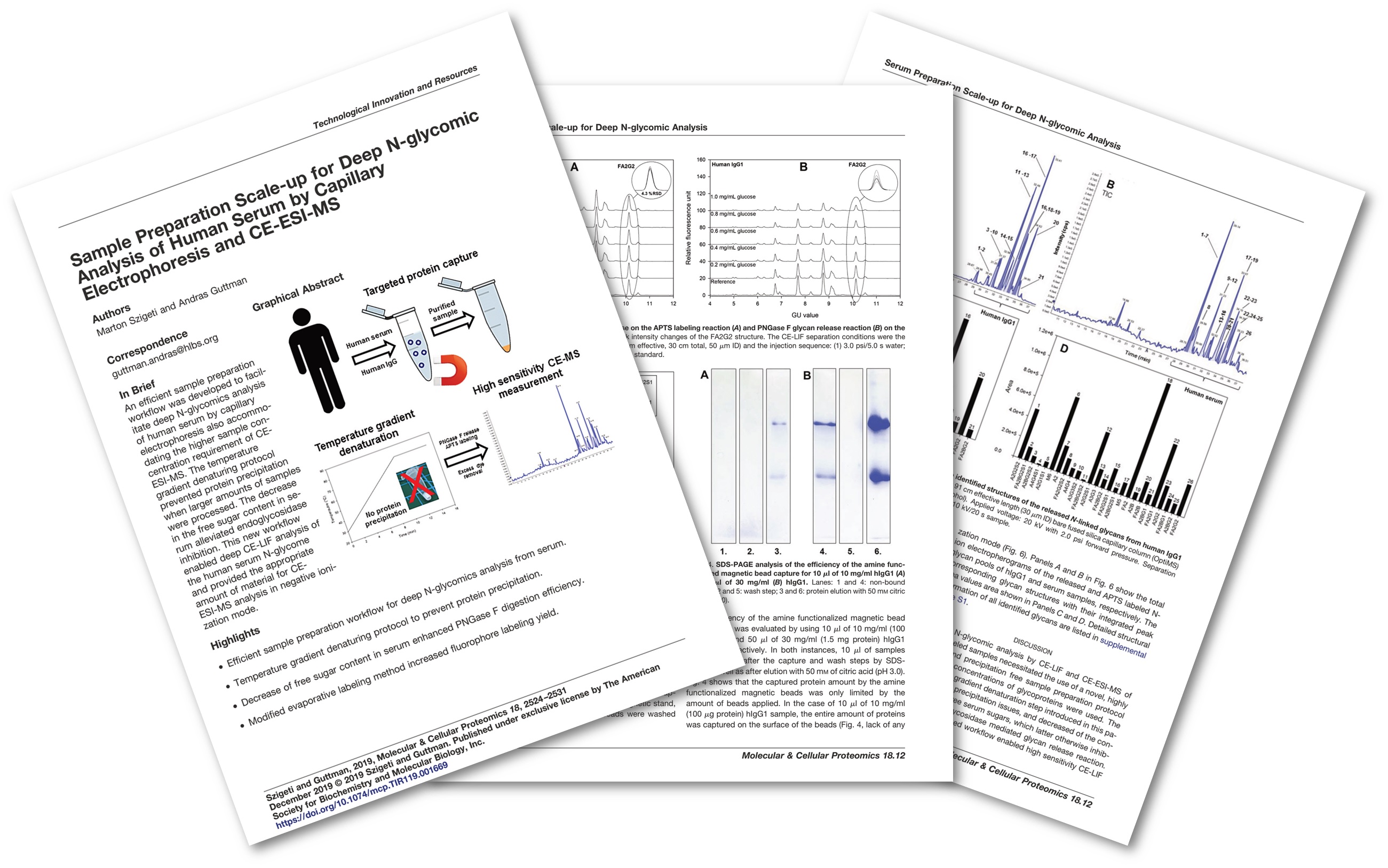
Example 2 – Automated carbohydrate sequencing
In addition to high resolution glycosylation profiling, carbohydrate sequencing is an important part of bioprocess analysis, especially when clients have unknown peaks in their sample. In glycan sequencing, carbohydrates are sequentially digested by various linkage-specific exoglycosidase enzymes allowing complete, high confidence structural elucidation for biological-relevance exploration or structural identity confirmation. Our in-house developed and validated semi- and fully automated sequencing methods ensure robust and fast service not available from other companies.
Sci Rep. 2017 Sep 15;7(1):11663
Example 3 – Purity and heterogeneity measurements & demonstrative measurement results
Precise determination of low-level impurities of biologics is a challenging bioanalytical task through the entire biologics and biosimilar development pipeline. Biopharma analytical laboratories require quantitative and validated data, which is exactly what Applied Bio-Analytics offers via utilization of the CE-SDS-MW method, which allows for the resolution of reduced and non-reduced mAb’s by size and to subsequently quantify the heterogeneity and impurities that may be present in mAb preparations. High quality CE-SDS data provides the critical confidence required for right decision-making – not necessarily available using other technologies. High reproducibility of our CE-SDS-MW measurement was confirmed by a 6-day trial with six runs per day using our internal standard monoclonal antibody sample. Our optimized methods offer excellent relative standard deviation (RSD) values for HC peak %Corrected area (under 0.2%), which is below the RSD value reported by the vendor of the kit used (see Figure 1 and Table 1).
Anal Chim Acta. 2021 Jun 29;1166:338492

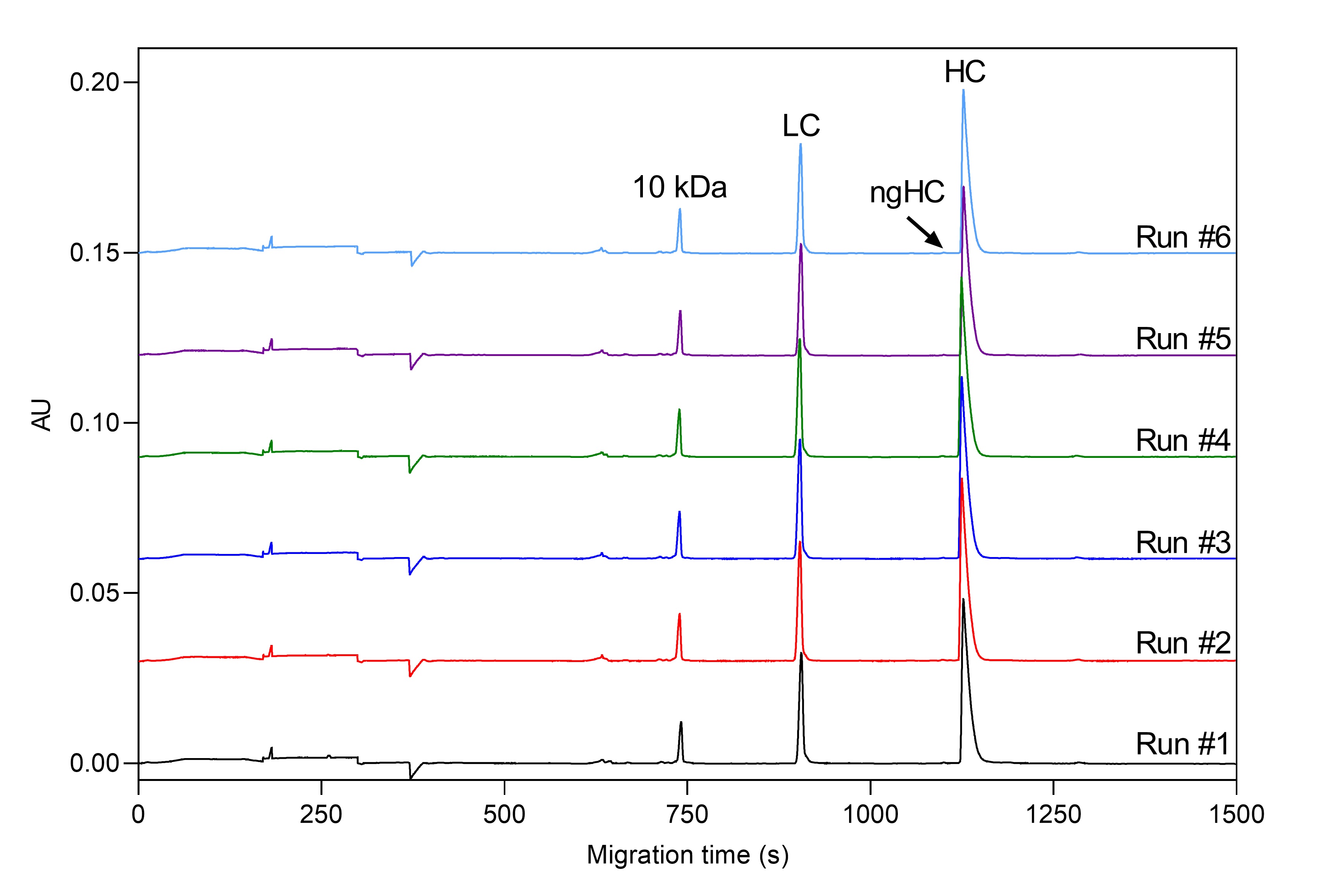
Figure 1. SDS-MW separation of Daratumumab mAb sample
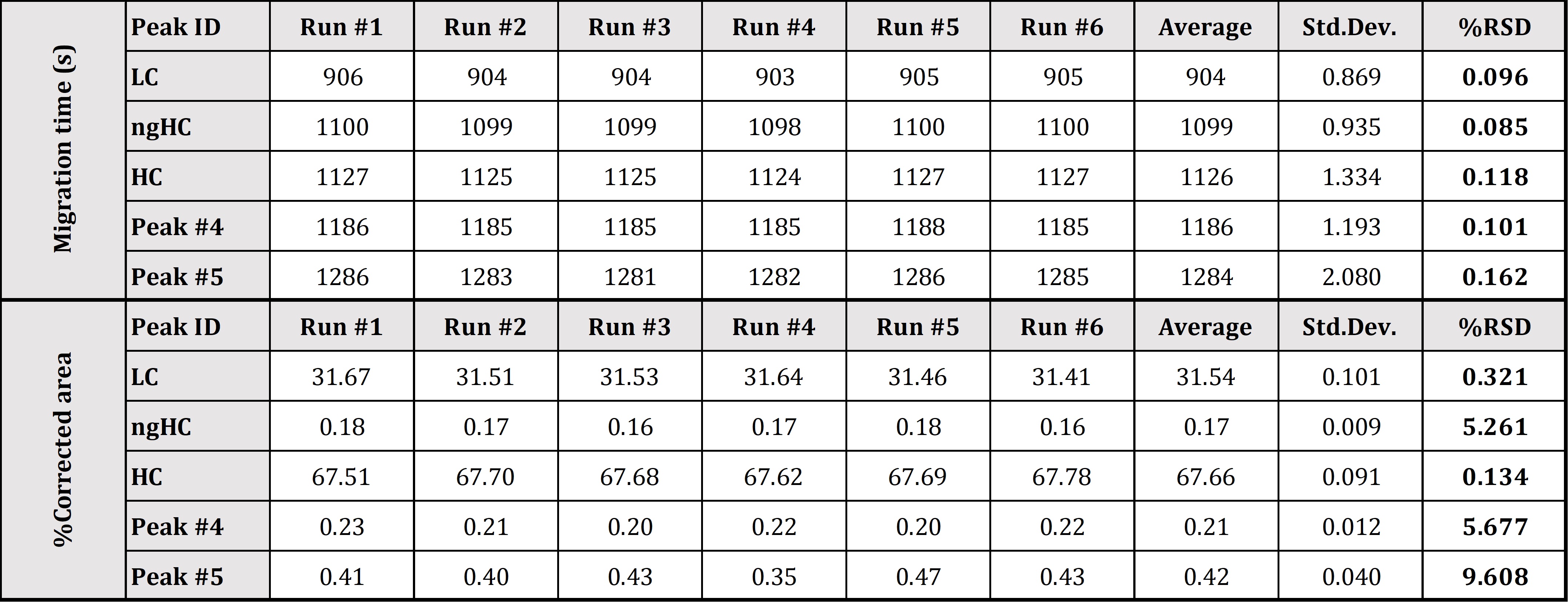
Table 1. Statistical evaluation of SDS-MW separation of Daratumumab mAb sample
Example 4 – Charge variant and charge heterogeneity analysis & demonstrative measurement results
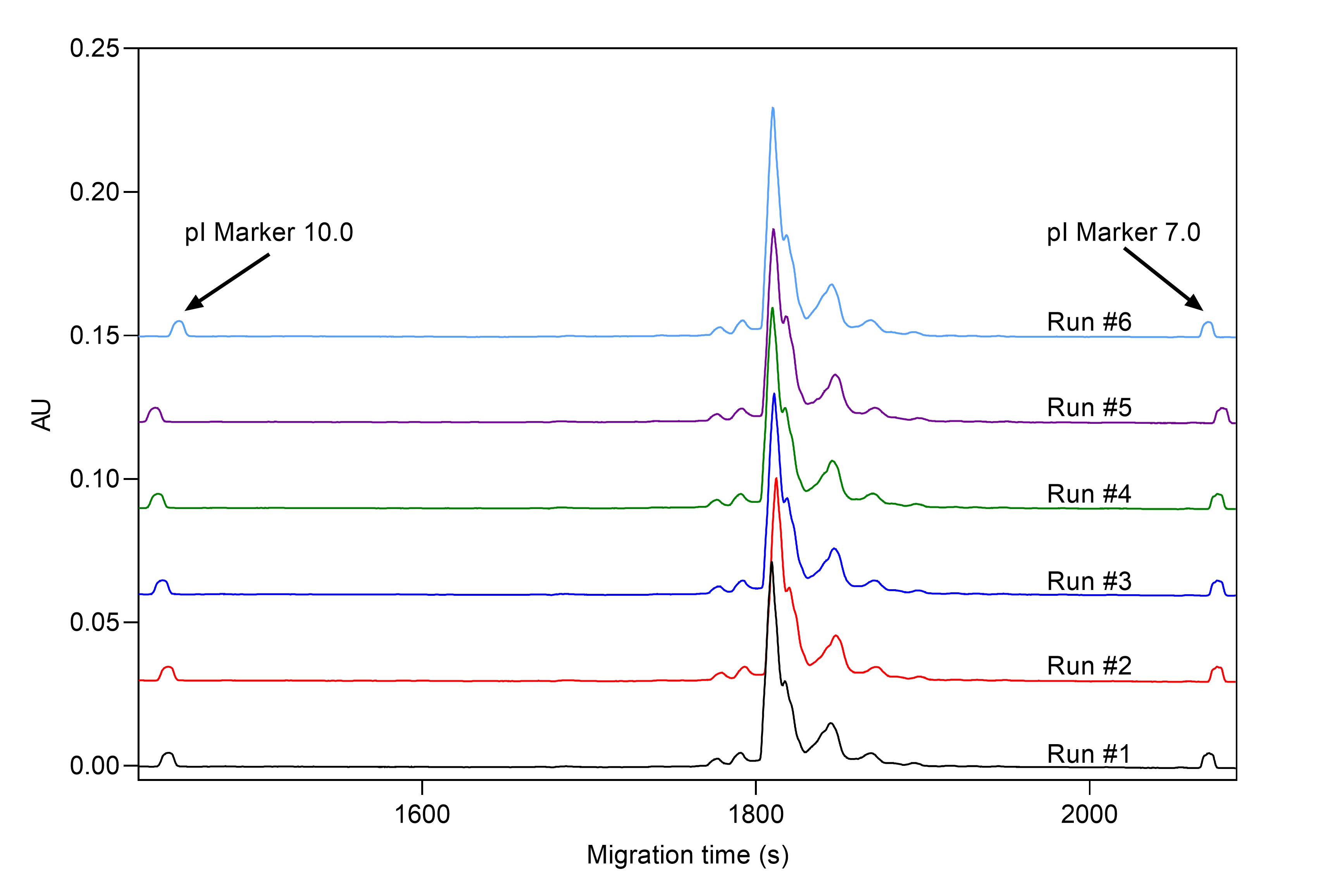
Working with biologics, especially with mAbs, requires information on different charge variants to determine identity and stability. Post-production analysis by cIEF can give information about purity, stability, and post-translational modifications of therapeutic proteins. By capillary isoelectric focusing (cIEF) we offer our clients ultra-high resolution charge heterogeneity data. cIEF is inherently consistent allowing global assessment, which is critical to both R&D and meeting regulatory requirements (see Figure 2 and Table 2).
Figure 1. cIEF analysis of Daratumumab mAb sample with CE
Food industry
We understand that food products are composed of a large variety of molecules with different chemical properties. Unlike other frequently used analytical techniques, our team of experts offer our clients in the food and beverage industry unique services utilizing the unmatched advantages of capillary electrophoresis. Applications where we can add the most to a project’s success:
-
Component analysis
-
Carbohydrate analysis
-
Protein and amino acid analysis
-
Water-soluble vitamin analysis
-
Ion analysis
-
-
Varietal identification
-
Quality assessment
-
Additive analysis
-
Analysis of natural toxins
-
Residue analysis including pesticides and antibiotics
We serve our clients with excellent quality data so that their resources can be used for the development / and production of their product, allowing clients to move forward with confidence at what they do best.
Example 1 – oligosaccharide and amino acid profiling of beers with capillary electrophoresis
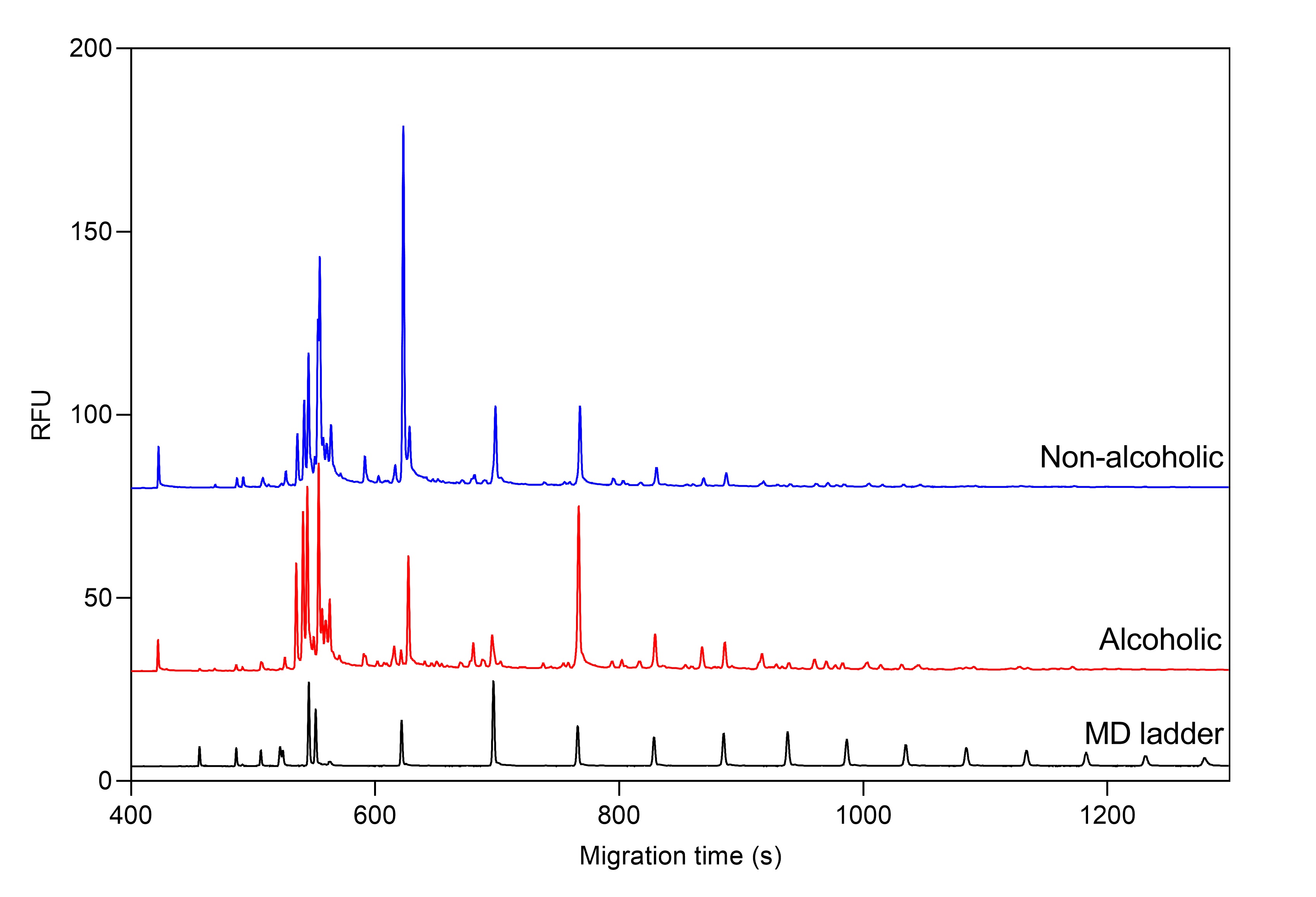
Figure 1. Total oligosaccharide profile of alcoholic and non-alcoholic beers by CE
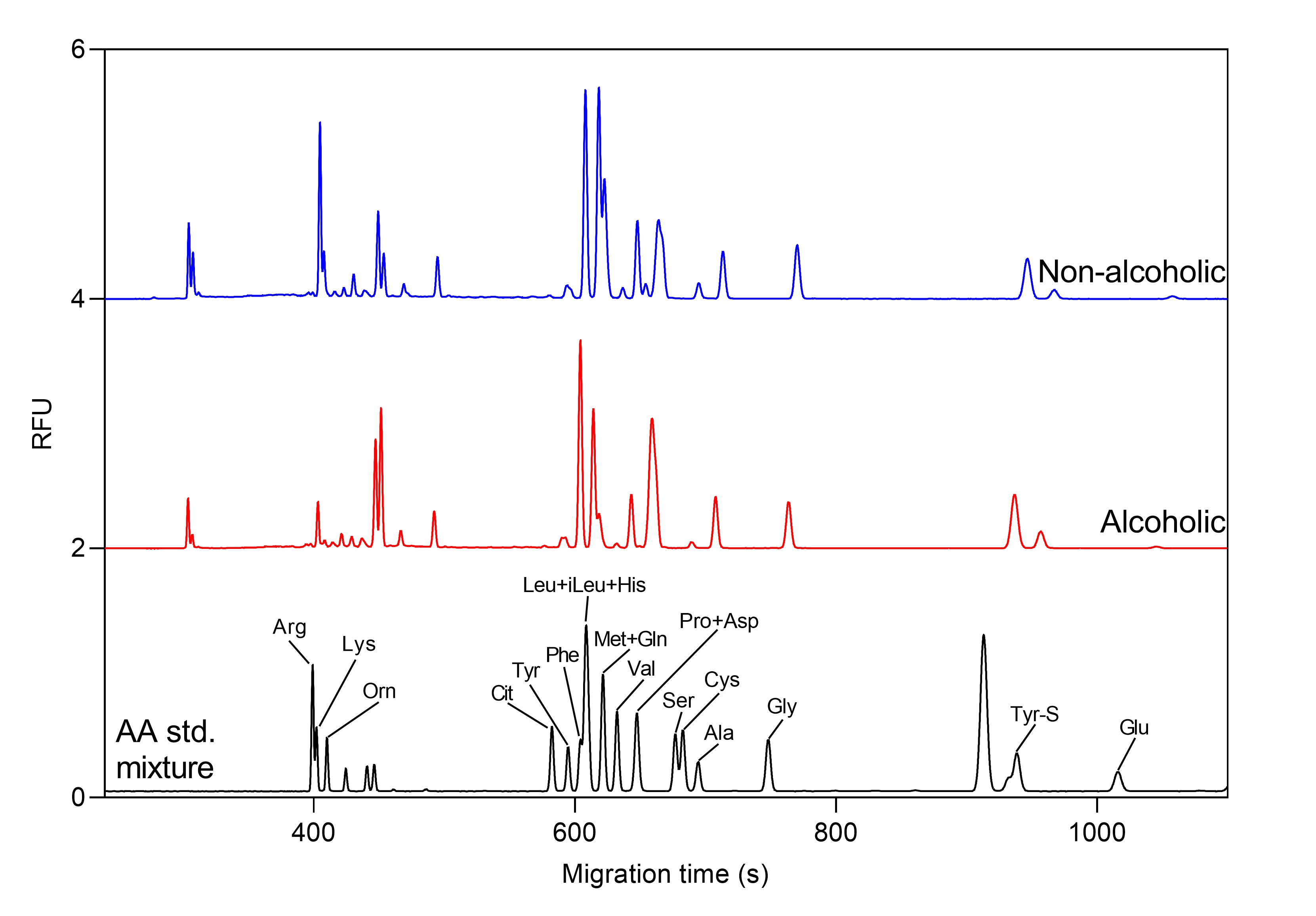
Figure 2. Total amino acid profile of alcoholic and non-alcoholic beers by CE
